Awardee of 2024 Joseph Altman Award in Developmental Neuroscience, Shawn Sorrells
Shawn Sorrells, Ph.D.
University of Pittsburgh, Department of Neuroscience
Assistant Professor
University of Pittsburgh, Department of Neuroscience
Assistant Professor
I am truly honored to receive the 2024 Joseph Altman award in Developmental Neuroscience. I extend my gratitude to Dr. Shirley Bayer for establishing this award and to the selection committee for choosing our work for recognition. I would like to use this opportunity to highlight the generous support, time, and contributions of my network of mentors, trainees, and colleagues. I was first introduced to Dr. Altman and Dr. Bayer’s research at the beginning of my postdoc and it inspired my deep interest in developmental neuroscience in ways I will describe below:
During my postdoc with Dr. Arturo Alvarez-Buylla, I became fascinated by how much (or little) of what we study in animal models translates to humans. Together with Dr. Mercedes Paredes, I began investigating neural stem cells and immature neurons in the embryonic, infant, childhood, and adult human temporal lobe. Inspired by Dr. Altman’s confrontation of dogma, we published our controversial finding that neurogenesis declines during childhood and is rare or absent in the adult human hippocampus (Sorrells et al., 2018, Paredes et al., 2018). Since then, new investigations have corroborated our observations of limited proliferation and an absence of a neurogenic niche in adults (Franjic et al. 2022, Zhou et al. 2022); however, it is still unknown why the timing for neurogenesis is different in humans and what other mechanisms of plasticity have been adopted instead. These questions are important for understanding the cellular basis of human memory and links to disorders like epilepsy or depression.
Strikingly, in many of the same adult brains lacking newborn neurons in the hippocampus, we found a large population of immature neurons in the amygdala. In collaboration with Dr. Vicente Herranz-Perez in the lab of Dr. Jose Manuel Garcia-Verdugo, we determined that despite their immature appearance, these neurons are born during gestation and delay their growth until postnatal life (Sorrells et al., 2019). When I opened my own lab, little was known about these neurons (Page et al., 2022), but my trainee Pia Alderman discovered that mice have a homologous region of their amygdala, opening up many experimental possibilities (Alderman et al., 2024). Together with Dave Saxon, a student in the lab of our collaborators Dr. Joshua Corbin and Dr. Stefano Vicini, we confirmed that these amygdala neurons develop surprisingly late, despite being born at typical embryonic ages. This work opens a new area of investigation into why these neurons extend their development into childhood and what their impact is on amygdala function during adolescence.
In their pioneering human developmental brain atlases, Altman and Bayer occasionally punctuate their regional identifications with question marks. This willingness to draw attention to uncertainty is a gracious reminder that it is the brain, not us, that holds the answers. Their careful work has pointed us toward uncovering new ages and regions where significant neuron structural growth happens in humans after we are born. Working with Dr. Marcos Assis Nascimento in Dr. Arturo Alvarez-Buylla’s lab, my trainees Sean Biagiotti and Samara Santiago recently uncovered a new migratory route in the human infant brain which sends immature interneurons to the entorhinal cortex (EC) until between 2-3 years of age (Nascimento et al. 2024). It will be very interesting to learn the role of these late-arriving interneurons in the plasticity of these memory centers in the brain.
As Dr. Altman first showed us decades ago, the brain is not complete by the time we are born. It is exciting to think about how our brain structure is influenced by life experiences, and to imagine new ways this might occur as we continue to uncover how the human brain changes throughout life.
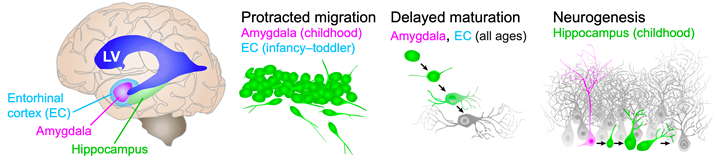
Figure legend: Cellular plasticity mechanisms in the human temporal lobe. Cartoon of the human brain viewed from the side showing the approximate locations of the internal structures relative to the lateral ventricle (LV, blue). Entorhinal cortex (EC, cyan), amygdala (magenta), and hippocampus (green). Three different cellular plasticity mechanisms are diagrammed on the right: Neural progenitors (magenta), immature neurons (green) and mature neurons (grey). Protracted migration appears in the EC from birth to toddler ages and in the amygdala into childhood. Delayed maturation occurs in the amygdala and EC throughout life. Neurogenesis is evident in the infant hippocampus and declines during childhood.

Shawn Sorrells, Ph.D.
Educational background
2000-2004:
B.A., in Cellular and Molecular Biology, with research honors - Cornell University, Ithaca NY, USA
2004-2011:
Ph.D. in Biological Sciences - Stanford University, Stanford CA, USA
Work experience
2011-2017:
Postdoctoral Scholar, University of California San Francisco, San Francisco CA, USA.
2017-2019:
Associate Specialist, University of California San Francisco, San Francisco CA, USA.
2019-present:
Assistant Professor, University of Pittsburgh, Pittsburgh PA, USA.





